

Journal of Surgery Concepts & Practice ›› 2023, Vol. 28 ›› Issue (05): 469-476.doi: 10.16139/j.1007-9610.2023.05.13
• Original article • Previous Articles Next Articles
LI Yuefeng1, HONG Jin2, LI Zhian1, RUAN Guodong1( ), CHEN Weiguo2(
), CHEN Weiguo2( )
)
Received:2022-09-28
Online:2023-09-25
Published:2024-01-04
CLC Number:
LI Yuefeng, HONG Jin, LI Zhian, RUAN Guodong, CHEN Weiguo. Prognostic analysis of the patients with HER2-positive breast cancer adjuvant treated with trastuzumab: a report of 1 246 cases[J]. Journal of Surgery Concepts & Practice, 2023, 28(05): 469-476.
Tab 1
Clinicopathological characteristics and treatment regimens[n(%)]
| Items | Total(%) (n=1 246) | lymph node metastasis | P value | |
|---|---|---|---|---|
| No (n=776) | Yes (n=470) | |||
| Age | 0.363 | |||
| <50 | 460(36.9) | 294(37.9) | 166(35.5) | |
| ≥50 | 786(63.1) | 482(62.1) | 304(64.7) | |
| Menstrual state | 0.572 | |||
| Premenopause | 519(41.7) | 328(42.3) | 191(40.6) | |
| Postmenopaus | 727(58.3) | 448(57.7) | 279(59.4) | |
| Malignancy history | 0.785 | |||
| Yes | 42(3.4) | 27(3.5) | 15(3.2) | |
| No | 1 204(96.6) | 749(96.5) | 455(96.8) | |
| Surgical approach | <0.001 | |||
| Breast-conserving | 263(21.1) | 201(25.9) | 62(13.2) | |
| Total mastectomy | 983(78.9) | 575(74.1) | 408(86.8) | |
| Pathology | <0.001 | |||
| DCIS with microinfiltration | 45(3.6) | 40(5.2) | 5(1.1) | |
| IDC | 1172(97.7) | 712(91.8) | 460(97.9) | |
| others | 29(2.3) | 24(3.1) | 5(1.1) | |
| pT stage | <0.001 | |||
| 1 | 646(51.8) | 470(60.6) | 176(37.4) | |
| 2 | 576(46.2) | 304(39.2) | 272(57.9) | |
| 3-4 | 24(1.9) | 2(0.3) | 22(4.7) | |
| Molecular typing | 0.790 | |||
| HER2 overexpression type | 637(51.1) | 399(51.4) | 238(50.6) | |
| Luminal B((HER2+) | 609(48.9) | 377(48.6) | 232(49.4) | |
| Histological grade | 0.048 | |||
| Ⅰ-Ⅱ | 441(35.4) | 285(36.7) | 156(33.2) | |
| Ⅲ | 712(57.1) | 425(54.8) | 287(61.1) | |
| NA | 93(7.5) | 66(8.5) | 27(5.7) | |
| Chemotherapy | <0.001 | |||
| A&T | 888(71.3) | 456(58.8) | 432(91.9) | |
| T&O | 358(28.7) | 320(41.2) | 38(8.1) | |
| Radiotherapy | <0.001 | |||
| Yes | 657(52.7) | 230(29.6) | 427(90.9) | |
| No | 589(47.3) | 546(70.4) | 43(9.1) | |
| Endocrine therapy | 0.483 | |||
| Yes | 623(50.0) | 382(49.2) | 241(51.3) | |
| No | 623(50.0) | 394(50.8) | 229(48.7) | |
Tab 2
Univariate analysis of factors influencing BCFI and OS in the breast cancer patients
| Feature | Univariate analysis(BCFI) | Univariate analysis(OS) |
|---|---|---|
| P value | P value | |
| pT stage | <0.001 | 0.036 |
| pN stage | <0.001 | 0.006 |
| Molecular typing | 0.001 | 0.182 |
| Chemotherapy | <0.001 | 0.208 |
| Endocrine therapy | <0.001 | 0.073 |
| Radiotherapy | <0.001 | 0.209 |
| Age | 0.313 | 0.302 |
| Menstrual state | 0.827 | 0.616 |
| History of malignancy | 0.225 | 0.330 |
| Surgical approach | 0.955 | 0.474 |
| Pathology | 0.154 | 0.483 |
| Histological grade | 0.518 | 0.896 |
Tab 3
Multivariate analysis of factors influencing BCFI and OS in the breast cancer patients
| Feature | Multivariate analysis(BCFI) | Multivariate analysis(OS) | |||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| pT stage | |||||||
| 1 | 1 | 1 | |||||
| 2-3 | 2.10 | 1.29-3.40 | 0.003 | 1.69 | 0.70-4.06 | 0.244 | |
| pN stage | |||||||
| 0 | 1 | 1 | |||||
| 1 | 1.28 | 0.73-2.23 | 0.390 | 1.81 | 0.64-5.09 | 0.261 | |
| 2-3 | 2.81 | 1.71-4.61 | <0.001 | 4.48 | 1.86-10.82 | 0.001 | |
| Molecular typing | - | ||||||
| HER2 overexpression type | 1 | ||||||
| Luminal B(HER2+) | 1.03 | 0.39-2.77 | 0.948 | ||||
| Chemotherapy | - | ||||||
| A&T | 1 | ||||||
| T&O | 0.40 | 0.18-0.85 | 0.017 | ||||
| Endocrine therapy | - | ||||||
| Yes | 1 | ||||||
| No | 2.50 | 1.59-3.94 | <0.001 | ||||
| Radiotherapy | - | ||||||
| Yes | 1 | ||||||
| No | 0.67 | 0.37-1.22 | 0.188 | ||||
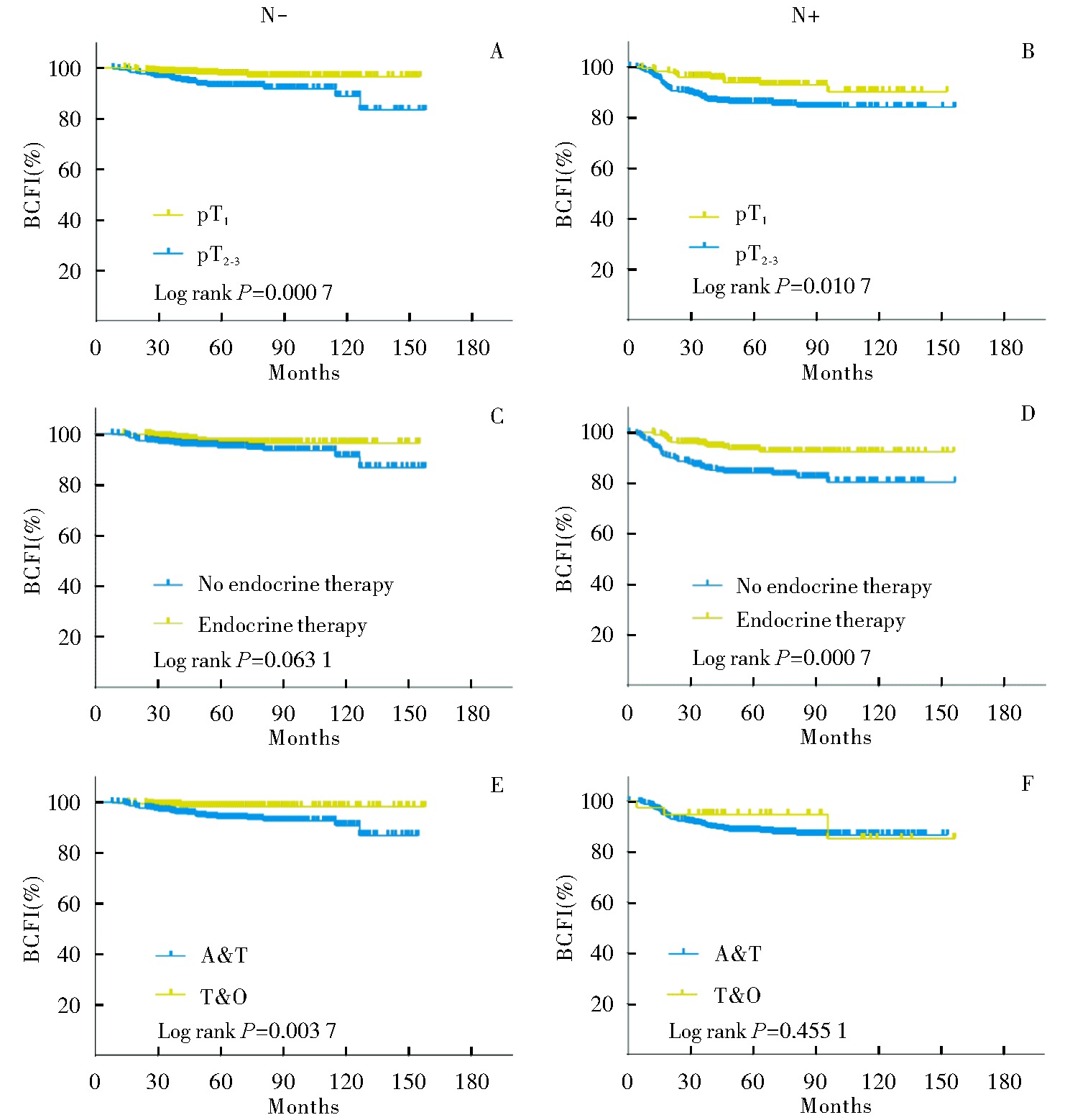
Fig 3
Subgroup analysis of BCFI in the HER2-positive breast cancer patients receiving trastuzumab monotherapy A: BCFI in patients with lymph node-negative disease stratified by different pT stages; B: BCFI in patients with lymph node-positive disease stratified by different pT stages; C: BCFI in lymph node-negative patients stratified by whether they received endocrine therapy; D: BCFI in lymph node-positive patients stratified by whether they received endocrine therapy; E: BCFI in lymph node-negative patients receiving different chemotherapy regimens; F: BCFI in lymph node-positive patients receiving different chemotherapy regimens.
Tab 4
Univariate and multivariate analysis of factors affecting BCFI in the lymph node-negative patients
| Feature | Univariate analysis P value | Multivariate analysis | ||
|---|---|---|---|---|
| HR | 95%CI | P value | ||
| pT | 0.003 | |||
| 1 | 1 | |||
| 2-3 | 2.36 | 1.15-4.88 | 0.020 | |
| Surgical approach | 0.031 | |||
| Breast-conserving | 1 | |||
| Total mastectomy | 0.43 | 0.22-0.87 | 0.019 | |
| Radiotherapy | 0.041 | |||
| Yes | 1 | |||
| No | 1.32 | 0.44-3.95 | 0.624 | |
| Chemotherapy | 0.004 | |||
| A&T | 1 | |||
| T&O | 0.35 | 0.13-0.91 | 0.032 | |
| Endocrine therapy | 0.063 | |||
| Yes | 1 | |||
| No | 2.19 | 1.06-4.52 | 0.034 | |
Tab 5
Univariate and multivariate analysis of factors affecting BCFI in lymph node-positive patient
| Feature | Univariate analysis P value | Multivariate analysis | ||
|---|---|---|---|---|
| HR | 95%CI | P value | ||
| pT | 0.011 | |||
| 1 | 1 | |||
| 2-3 | 2.28 | 1.20-4.32 | 0.012 | |
| Endocrine therapy | 0.001 | |||
| Yes | 1 | |||
| No | 2.65 | 1.48-4.74 | 0.001 | |
| Molecular typing | 0.002 | |||
| HER2 overexpression type | 1 | |||
| Luminal B(HER2+) | 0.88 | 0.22-3.54 | 0.860 | |
| [1] |
LIN L, LI Z, YAN L, et al. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990-2019[J]. J Hematol Oncol, 2021, 14(1):197.
doi: 10.1186/s13045-021-01213-z |
| [2] | CAO W, CHEN H D, YU Y W, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J (Engl), 2021, 134(7):783-791. |
| [3] | XIA C, DONG X, LI H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants[J]. Chin Med J (Engl), 2022, 135(5):584-590. |
| [4] |
HAYES D F. HER2 and breast cancer - a phenomenal success story[J]. N Engl J Med, 2019, 381(13):1284-1286.
doi: 10.1056/NEJMcibr1909386 URL |
| [5] | WENG Y, LIANG W, JI Y, et al. Key genes and prognostic analysis in HER2+ breast cancer[J]. Technol Cancer Res Treat, 2021, 20:1533033820983298. |
| [6] | PRAT A, PINEDA E, ADAMO B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer[J]. Breast, 2015, 24 (Suppl 2):S26-S35. |
| [7] |
BLACKWELL K, GLIGOROV J, JACOBS I, et al. The global need for a trastuzumab biosimilar for patients with HER2-positive breast cancer[J]. Clin Breast Cancer, 2018, 18(2):95-113.
doi: S1526-8209(17)30406-8 pmid: 29525430 |
| [8] | 中国抗癌协会乳腺癌专业委员会. 中国抗癌协会乳腺癌诊治指南与规范(2021年版)[J]. 中国癌症杂志, 2021, 31(10):954-1040. |
| Breast Cancer Expert Committee of China Anti-Cancer Association. China Anti-Cancer Association breast cancer diagnosis and treatment guidelines and standards (2021 edition)[J]. China Oncology, 2021, 31(10):954-1040. | |
| [9] |
GION M, TRAPANI D, CORTES A, et al. Systemic therapy for HER2-positive metastatic breast cancer: moving into a new era[J]. Am Soc Clin Oncol Educ Book, 2022, 42:1-11.
doi: 10.1200/EDBK_351222 pmid: 35671434 |
| [10] |
MAXIMIANO S, MAGALHAES P, GUERREIRO M P, et al. Trastuzumab in the treatment of breast cancer[J]. BioDrugs, 2016, 30(2):75-86.
doi: 10.1007/s40259-016-0162-9 pmid: 26892619 |
| [11] |
SLAMON D, EIERMANN W, ROBERT N, et al. Adjuvant trastuzumab in HER2-positive breast cancer[J]. N Engl J Med, 2011, 365(14):1273-1283.
doi: 10.1056/NEJMoa0910383 URL |
| [12] |
CAMERON D, PICCART-GEBHART M J, GELBER R D, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial[J]. The Lancet, 2017, 389(10075):1195-1205.
doi: 10.1016/S0140-6736(16)32616-2 URL |
| [13] |
DERAKHSHANI A, REZAEI Z, SAFARPOUR H, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy[J]. J Cell Physiol, 2020, 235(4):3142-3156.
doi: 10.1002/jcp.29216 pmid: 31566722 |
| [14] |
BRASO-MARISTANY F, GRIGUOLO G, PASCUAL T, et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade[J]. Nat Commun, 2020, 11(1):385.
doi: 10.1038/s41467-019-14111-3 |
| [15] |
ROBSON D, VERMA S. Anthracyclines in early-stage breast cancer: is it the end of an era?[J]. Oncologist, 2009, 14(10):950-958.
doi: 10.1634/theoncologist.2008-0070 pmid: 19561291 |
| [16] |
ANAMPA J, MAKOWER D, SPARANO J A. Progress in adjuvant chemotherapy for breast cancer: an overview[J]. BMC Med, 2015, 13:195.
doi: 10.1186/s12916-015-0439-8 pmid: 26278220 |
| [17] |
DAVIDSON A, GELMON K. Do anthracyclines still have a role in adjuvant chemotherapy of breast cancer?[J]. Future Oncol, 2011, 7(1):37-55.
doi: 10.2217/fon.10.163 pmid: 21174537 |
| [18] |
DE GLAS N, BASTIAANNET E, DE BOER A, et al. Improved survival of older patients with advanced breast cancer due to an increase in systemic treatments: a population-based study[J]. Breast Cancer Res Treat, 2019, 178(1):141-149.
doi: 10.1007/s10549-019-05356-z |
| [19] |
TOGNELA A, BEITH J, KIELY B, et al. Small HER2-positive breast cancer: should size affect adjuvant treatment?[J]. Clin Breast Cancer, 2015, 15(4):277-284.
doi: 10.1016/j.clbc.2014.12.012 pmid: 25676930 |
| [20] | LI J, JIANG Z. Chinese Society of Clinical Oncology Breast Cancer (CSCO BC) guidelines in 2022: stratification and classification[J]. Cancer Biol Med, 2022, 19(6):769-773. |
| [21] |
BANERJEE S, SMITH I E. Management of small HER2-positive breast cancers[J]. Lancet Oncol, 2010, 11(12):1193-1199.
doi: 10.1016/S1470-2045(10)70119-4 pmid: 21126688 |
| [22] |
PEROTTI A, CRESTA S, GRASSELLI G, et al. Cardiotoxic effects of anthracycline-taxane combinations[J]. Expert Opin Drug Saf, 2003, 2(1):59-71.
pmid: 12904125 |
| [23] |
GREENE J, HENNESSY B. The role of anthracyclines in the treatment of early breast cancer[J]. J Oncol Pharm Pract, 2015, 21(3):201-212.
doi: 10.1177/1078155214531513 pmid: 24769570 |
| [24] |
BARROSO-SOUSA R, EXMAN P, TOLANEY S M. De-escalating treatment in the adjuvant setting in HER2-positive breast cancer[J]. Future Oncol, 2018, 14(10):937-945.
doi: 10.2217/fon-2017-2500 URL |
| [25] |
TOLANEY S M, BARRY W T, DANG C T, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer[J]. N Engl J Med, 2015, 372(2):134-141.
doi: 10.1056/NEJMoa1406281 URL |
| [26] | CASTRELLON A B, GLÜCK S. Adjuvant therapy for HER2 positive breast cancer: are anthracyclines still ne-cessary?[J]. Clin Adv Hematol Oncol, 2008, 6(9):666-672. |
| [27] |
VAN RAMSHORST M S, VAN DER VOORT A, VAN WERKHOVEN E D, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2018, 19(12):1630-1640.
doi: S1470-2045(18)30570-9 pmid: 30413379 |
| [28] |
MAUGHAN K L, LUTTERBIE M A, HAM P S. Treatment of breast cancer[J]. Am Fam Physician, 2010, 81(11):1339-1346.
pmid: 20521754 |
| [29] |
ALMAHARIQ M F, QUINN T J, SIDDIQUI Z, et al. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer[J]. Radiother Oncol, 2020, 142:186-194.
doi: S0167-8140(19)33107-X pmid: 31615634 |
| [30] |
SINNADURAI S, KWONG A, HARTMAN M, et al. Breast-conserving surgery versus mastectomy in young women with breast cancer in Asian settings[J]. BJS Open, 2019, 3(1):48-55.
doi: 10.1002/bjs5.2019.3.issue-1 URL |
| [31] |
MIRICESCU D, TOTAN A, STANESCU-SPINU II, et al. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects[J]. Int J Mol Sci, 2020, 22(1):173.
doi: 10.3390/ijms22010173 URL |
| [1] | YANG Yi, YANG Xingxia, JIN Sili, ZHANG Xu, ZHU Juanying, CHEN Xiaosong. Clinical application of preoperative MRI examination in breast-conserving surgery for ductal carcinoma in situ [J]. Journal of Surgery Concepts & Practice, 2023, 28(04): 378-382. |
| [2] | LIU Yingting, YI Hongmei, WANG Xue, YANG Chunxue, OUYANG Binshen XU Haimin, WANG Chaofu. Clinicopathological features and prognosis of 17 cases of duodenal-type follicular lymphoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 362-368. |
| [3] | ZHANG Lanlan, YANG Qiao, NIE Zunzhen, GUO Ying. Thoracic SMARCA4-deficient undifferentiated tumour: a case report [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 389-392. |
| [4] | HU Jingjing, SHEN Yinzhong, LIU Li, LU Hongzhou. Current status and research progress of diagnosis and treatment of AIDS with disseminated non-tuberculous mycobacterial disease [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 402-406. |
| [5] | XU Li, GAO Huajie, YANG Mengge, LI Yue, JI Suqiong. Clinical characteristics of anti-SRP antibody positive immune-mediated necrotizing myopathy with anti-TRIM21/Ro52 antibody positive [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(03): 247-254. |
| [6] | ZHOU Xiaodie, CHEN Weiwei, YU Bo, WANG Xuan, WANG Jianjun, SHI Qunli, RAO Qiu, BAO Wei. Clinicopathological features of urothelial carcinoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(03): 292-299. |
| [7] | QIAO Minjie, ZHOU Wei, CHEN Yi. Role of serum high mobility group box-B1 in evaluating prognosis of sepsis [J]. Journal of Internal Medicine Concepts & Practice, 2023, 18(02): 70-75. |
| [8] | SONG Luqian, CHANG Chunkang. Interpretation of clinical practice guidelines for myelodysplastic syndrome (version 1, 2023) of National Comprehensive Cancer Nerwork(NCCN) [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(02): 116-120. |
| [9] | XU Jiankun, ZHOU Luting, ZHANG Wenjing, XU Haimin, WANG Chaofu. The prognostic value of CA9 expression in clear cell renal cell carcinoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(01): 37-43. |
| [10] | WANG Han, LU Haidi, WANG Lei, CONG Wenming, ZHENG Jianming, BAI Chenguang. Clinicopathological features of 2 cases of squamous cell carcinoma and 2 cases of adenosquamous carcinoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(01): 44-49. |
| [11] | LI Yongde, WANG Yang, LI Xiang, LI Wenjie, XIE Di, JIANG Shaowei, GE Xiaoli, WANG Hairong, GAO Chengjin, PAN Shuming. A retrospective study on prediction of neurological outcome in cardiac arrest patients of out-hospital [J]. Journal of Internal Medicine Concepts & Practice, 2022, 17(06): 447-452. |
| [12] | WANG Jin, GUO Rui, LI Biao, ZHANG Xiaozhe. Prognostic evaluation of extranodal natural killer/T-cell lymphoma, nasal type(ENKTL) with 18F-FDG PET/CT [J]. Journal of Diagnostics Concepts & Practice, 2022, 21(06): 702-709. |
| [13] | YANG Cuiyan, WANG Haoyu, CHEN Xiaosong, SHEN Kunwei. Study on tumour suppressor gene TP53 mutation and prognosis in patients with triple-negative breast cancer [J]. Journal of Surgery Concepts & Practice, 2022, 27(05): 421-428. |
| [14] | XIE Wen, LIANG Huaiyu, DONG Lei, YUAN Fei, WANG Chaofu, GUO Yan. Analysis of genetic status of pivotal driver genes in pancreatic ductal adenocarcinoma and their correlation with clinicopathologic features [J]. Journal of Diagnostics Concepts & Practice, 2022, 21(05): 581-587. |
| [15] | WANG Wenhan, XIA Shujun, ZHAN Weiwei. Application of long non-coding RNA ENST00000489676 detection in ultrasonographic evaluation of cervical lymph node metastasis in papillary thyroid carcinoma [J]. Journal of Diagnostics Concepts & Practice, 2022, 21(04): 514-519. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||