

Journal of Diagnostics Concepts & Practice ›› 2025, Vol. 24 ›› Issue (04): 431-440.doi: 10.16150/j.1671-2870.2025.04.010
• Original articles • Previous Articles Next Articles
LIN Liya, WU Xi, MAO Yinqi, CHEN Guangming, WU Wenman, DAI Jing, WANG Xuefeng, DING Qiulan( )
)
Received:2025-01-20
Revised:2025-02-25
Accepted:2025-03-25
Online:2025-08-25
Published:2025-09-09
Contact:
DING Qiulan
E-mail:qiulan_ding@126.com
CLC Number:
LIN Liya, WU Xi, MAO Yinqi, CHEN Guangming, WU Wenman, DAI Jing, WANG Xuefeng, DING Qiulan. Three disintegrin-like domain mutations of ADAMTS13: functional deficiency and association with thrombosis[J]. Journal of Diagnostics Concepts & Practice, 2025, 24(04): 431-440.
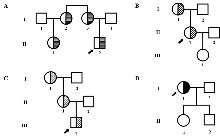
Figure 1
Pedigrees of four thrombotic patients carrying ADAMTS13 gene variants A: Pedigree 1. B: Pedigree 2. C: Pedigree 3. D: Pedigree 4. Legend: Circle (○): Female. Square (□): Male. Arrow (↗): Proband. Horizontal line: Carriers of the ADAMTS13 Pro301Ala heterozygous missense va-riant. Slash: Carriers of the ADAMTS13 Pro301Arg heterozygous missense variant. Black: Carriers of the ADAMTS13 Arg349Cys heterozygous missense variant.
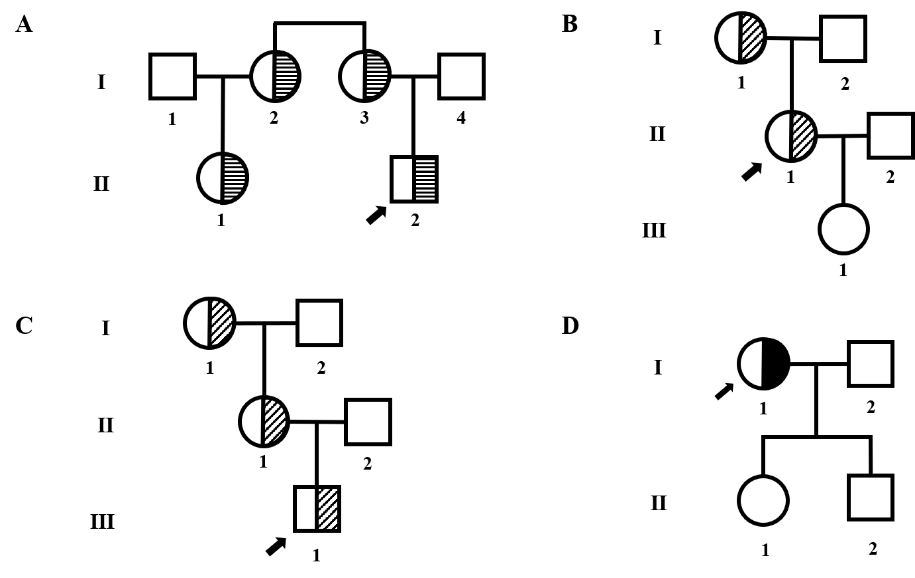
Table 1
Results of Gene Analysis and Clinical Phenotype
| Proband | Gene Analysis | ADAMTS13: Act(%) | ADAMTS13: Ag(%) | VWF:Act (%) | VWF:Ag (%) | VWF:CB /VWF:Ag(Ratio) | ADAMTS13:Act /VWF:Ag(Ratio) |
|---|---|---|---|---|---|---|---|
| 1 | ADAMTS13 c.901C>G:p.Pro301Ala* | 62.03 | 70.35 | 211.6 | 214.9 | 1.47 | 0.289 |
| 2 | ADAMTS13 c.902C>G:p.Pro301Arg* | 67.35 | 75.46 | 213.7 | 216.6 | 1.28 | 0.311 |
| 3 | ADAMTS13 c.902C>G:p.Pro301Arg* | 72.88 | 78.34 | 158.2 | 167.2 | 1.39 | 0.436 |
| 4 | ADAMTS13 c.1045C>T:p.Arg349Cys | 57.42 | 66.94 | 179.5 | 188.9 | 1.33 | 0.304 |
| Normal range | 60.00-150.00 | 40.00-120.00 | 50.0-150.0 | 50.0-150.0 | 0.70-1.20 | 0.500-2.000 |
Figure 2
Sanger sequencing results of probands from four pedigrees A: Normal individual showing wild-type sequence in exon 8 of the ADAMTS13 gene.B: Proband of Family 1 with a heterozygous ADAMTS13 mutation in exon 8: c.901C>G (p.Pro301Ala).C: Proband of Family 2 with a heterozygous ADAMTS13 mutation in exon 8: c.902C>G (p.Pro301Arg).D: Proband of Family 3 with the same heterozygous ADAMTS13 mutation in exon 8: c.902C>G (p.Pro301Arg).E: Normal individual showing wild-type sequence in exon 9 of the ADAMTS13 gene.F: Proband of Family 4 with a heterozygous ADAMTS13 mutation in exon 9: c.1045C>T (p.Arg349Cys).
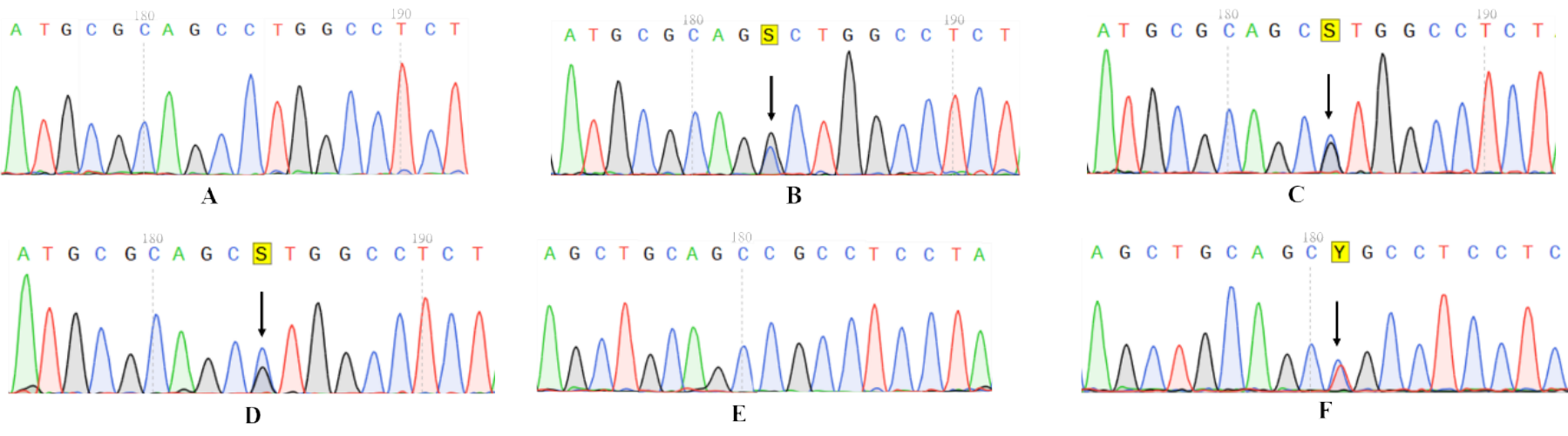
Figure 3
Results of vWF multimer analysis A: vWF polymer electrophoress. Lane 1 (NP): normal plasma; lanes 2-5 (P1-P4): plasma from 4 probands. B: Semi-quantitative analysis of the grayscale values of vWF polymer electrophoresis images. C: Semi-quantitative results of vWF HMWMs statistical analysis. HMWMs: High molecular weight polymers. IMWMs: Intermediate molecular weight polymers. LMWMs: Low molecular weight polymers. **: P<0.01 vs NP. ***: P<0.001 vs NP. ****: P<0.000 1 vs NP.
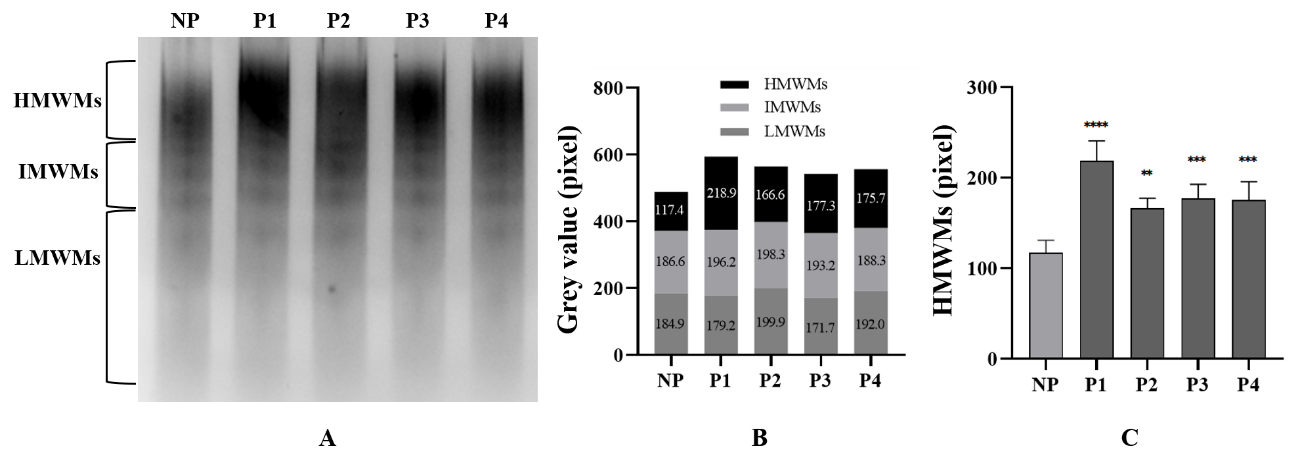
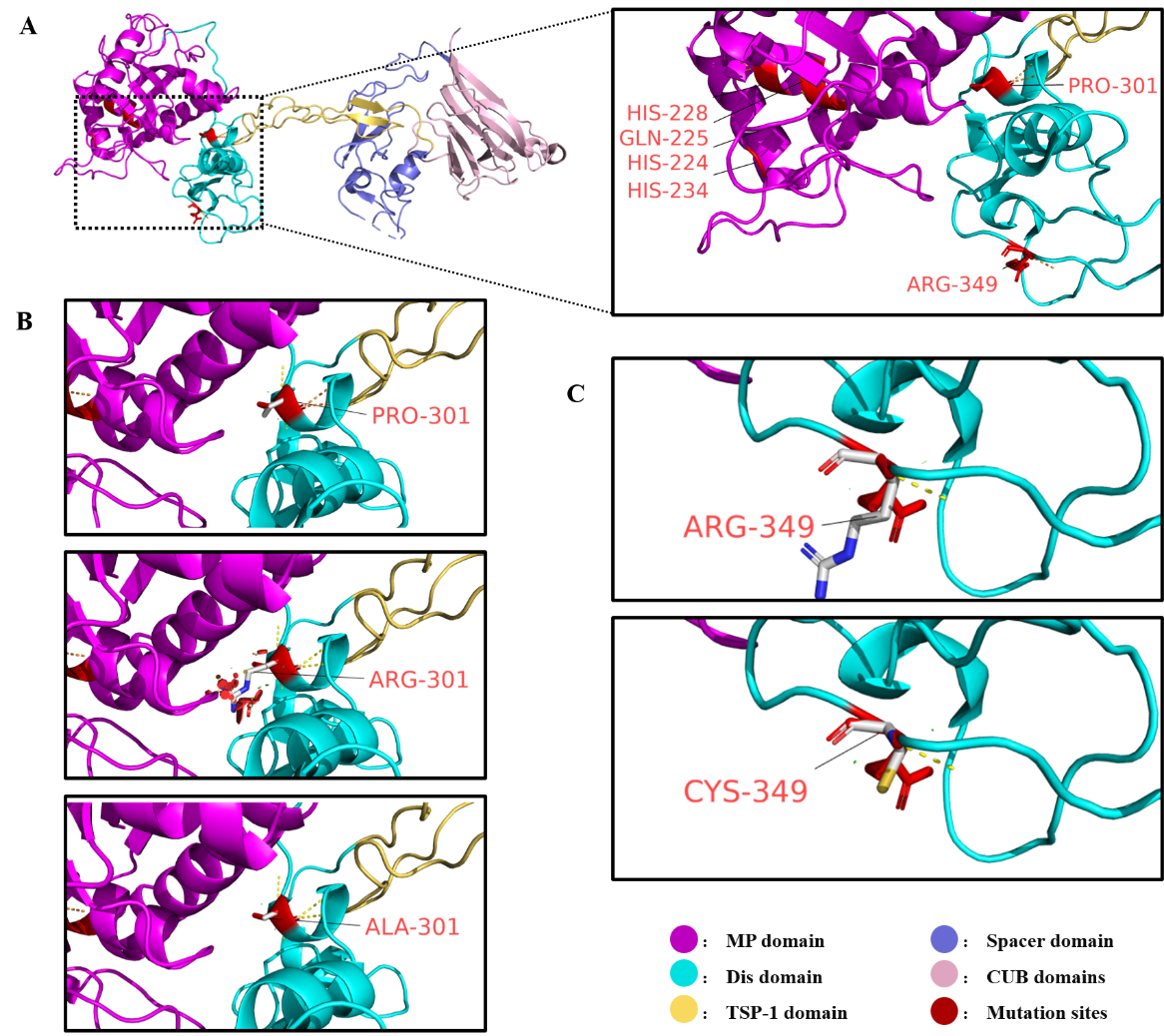
Figure 4
Two amino acid residues (Pro301 and Arg349) in the disintegrin-like domain of ADAMTS13 and the structural effects of their variants A: The structure of wild-type ADAMTS13 protein, with the catalytic active site (HIS-224, GLN-225, HIS-228, HIS-234) in the metalloprotease domain and the studied variant sites (PRO-301, ARG-349) highlighted in red. B: Structural comparison between wild-type ADAMTS13 residue PRO-301 and mutated residues ALA-301 and ARG-301 in ADAMTS13. The red disks surrounding the ARG-301 residue represent steric hindrance caused by the variant. C: Structural comparison between wild-type ADAMTS13 residue ARG-349 and mutated residue CYS-349 in ADAMTS13.
| [1] | LEVY G G, NICHOLS W C, LIAN E C, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura[J]. Nature, 2001, 413(6855):488-494. |
| [2] | SADLER J E. Biochemistry and genetics of von Wille-brand factor[J]. Annu Rev Biochem, 1998, 67:395-424. |
| [3] |
DE GROOT R, BARDHAN A, RAMROOP N, et al. Essential role of the disintegrin-like domain in ADAMTS13 function[J]. Blood, 2009, 113(22):5609-5616.
doi: 10.1182/blood-2008-11-187914 pmid: 19234142 |
| [4] |
FENG Y, LI X Y, XIAO J, et al. ADAMTS13: more than a regulator of thrombosis[J]. Int J Hematol, 2016, 104(5):534-539.
pmid: 27696191 |
| [5] |
ZHENG X, CHUNG D, TAKAYAMA T K, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura[J]. J Biol Chem, 2001, 276(44):41059-41063.
doi: 10.1074/jbc.C100515200 pmid: 11557746 |
| [6] | HOMMAIS A, RAYES J, HOULLIER A, et al. Molecular characterization of four ADAMTS13 mutations responsible for congenital thrombotic thrombocytopenic purpura (Upshaw-Schulman syndrome)[J]. Thromb Haemost, 2007, 98(3):593-599. |
| [7] | DE WAELE L, VERMEERSCH L, NGUYEN T T, et al. In vitro characterization of a novel Arg102 mutation in the ADAMTS13 metalloprotease domain[J]. J Thromb Haemost, 2023, 21(3):682-690. |
| [8] |
JIANG Y, HUANG D, KONDO Y, et al. Novel mutations in ADAMTS13 CUB domains cause abnormal pre-mRNA splicing and defective secretion of ADAMTS13[J]. J Cell Mol Med, 2020, 24(7):4356-4361.
doi: 10.1111/jcmm.15025 pmid: 32073234 |
| [9] |
丁秋兰, 王学锋. 遗传性易栓症的表型和基因诊断流程[J]. 诊断学理论与实践, 2019, 18(2):127-132.
doi: 10.16150/j.1671-2870.2019.02.002 |
| DING Q L, WANG X L. The phenotype and flowchart of gene diagnosis in inherited thrombophilia[J]. J Diagn Concepts Pract, 2019, 18(2):127-132. | |
| [10] | 李蕾, 吴希, 戴菁, 等. 中国118例颅内静脉窦血栓患者的临床特点及危险因素分析[J]. 诊断学理论与实践, 2023, 22(3):261-269. |
| LI L, WU X, DAI J, et al. Clinical characteristics and risk factor analysis of 118 patients with cerebral venous sinus thrombosis[J]. J Diagn Concepts Pract, 2023, 22(3):261-269. | |
| [11] | 李蕾, 吴希, 许冠群, 等. 基于新一代测序技术的易栓症基因检测Panel的建立及其在中国静脉血栓患者遗传背景研究中的临床应用[J]. 诊断学理论与实践, 2019, 18(4):394-401. |
| LI L, WU X, XU G Q, et al. Establishment and applica tion of thrombophilia gene detection Panel based on next generation sequencing in identification of genetic back ground of Chinese patients with venous thromboembolism[J]. J Diagn Concepts Pract, 2019, 18(4):394-401. | |
| [12] | LIANG Q, ZHANG Z, DING B, et al. A noncanonical spli-cing variant c.875-5 T > G in von Willebrand factor causes in-frame exon skipping and type 2A von Willebrand disease[J]. Thromb Res, 2024, 236:51-60. |
| [13] | 金佩佩, 梁茜, 戴菁, 等. 一例2N型遗传性血管性血友病家系的表型诊断和基因型分析[J]. 诊断学理论与实践, 2018, 17(2):151-154. |
| JIN P P, LIANG Q, DAI J, et al. Phenotype and genotype analysis of a Chinese pedigree with 2N type von Wille-brand disease[J]. J Diagn Concepts Pract, 2018, 17(2):151-154. | |
| [14] | LIANG Q, QIN H, DING Q L, et al. Molecular and clinical profile of VWD in a large cohort of Chinese population: application of next generation sequencing and CNVplex® technique[J]. Thromb Haemost, 2017, 117(8):1534-1548. |
| [15] |
BUDDE U, SCHNEPPENHEIM R, EIKENBOOM J, et al. Detailed von Willebrand factor multimer analysis in patients with von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease (MCMDM-1VWD)[J]. J Thromb Haemost, 2008, 6(5):762-771.
doi: 10.1111/j.1538-7836.2008.02945.x pmid: 18315556 |
| [16] | EDVARDSEN M S, HANSEN E-S, UELAND T, et al. Impact of the von Willebrand factor-ADAMTS-13 axis on the risk of future venous thromboembolism[J]. J Thromb Haemost, 2023, 21(5):1227-1237. |
| [17] | TAYLOR A, VENDRAMIN C, SINGH D, et al. von Wille-brand factor/ADAMTS13 ratio at presentation of acute ischemic brain injury is predictive of outcome[J]. Blood Adv, 2020, 4(2):398-407. |
| [18] | LANCELLOTTI S, SACCO M, TARDUGNO M, et al. The von Willebrand factor-ADAMTS-13 axis:a two-faced Janus in bleeding and thrombosis[J/OL]. 2022[2025-01-20]. https://www.btvb.org/btvb/article/view/11. |
| [19] | PHILIPPE A, GENDRON N, BORY O, et al. Von Wille-brand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: insight from vWF/ADAMTS13 ratio imbalance[J]. Angiogenesis, 2021, 24(3):407-411. |
| [20] |
AI J H, SMITH P, WANG S W, et al. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor[J]. J Biol Chem, 2005, 280(33):29428-29434.
doi: 10.1074/jbc.M505513200 pmid: 15975930 |
| [21] | FUJIMURA Y, MATSUMOTO M, KOKAME K, et al. Pregnancy-induced thrombocytopenia and TTP, and the risk of fetal death, in Upshaw-Schulman syndrome: a series of 15 pregnancies in 9 genotyped patients[J]. Br J Haematol, 2009, 144(5):742-754. |
| [22] | AKIYAMA M, TAKEDA S, KOKAME K, et al. Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor[J]. Proc Natl Acad Sci U S A, 2009, 106(46):19274-19279. |
| [23] | HASSENPFLUG W A, OBSER T, BODE J, et al. Genetic and functional characterization of ADAMTS13 variants in a patient cohort with upshaw-schulman syndrome investigated in Germany[J]. Thromb Haemost, 2018, 118(4): 709-722. |
| [1] | XIAO Jianwen, YI Weijia. Research progress on clinical application of anti-tissue factor pathway inhibitor in hemophilia [J]. Journal of Diagnostics Concepts & Practice, 2025, 24(02): 226-232. |
| [2] | YANG Mingkang, LIU Yu, XU Guanqun, WANG Jianbiao, WANG Xuefeng, LIANG Qian. Value of vWF-related indicators in the diagnosis of liver cirrhosis progression in patients with hepatitis B [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(06): 574-579. |
| [3] | ZHOU Lihua, SHEN Ru, QU Kexuan, WANG Aihua, CHEN Youhui, YUAN Zhimin. Study on the Bw11 subtype caused by the 695 T>C mutation in exon 7 of the ABO blood group gene [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(04): 392-397. |
| [4] | ZHU Weiwei, LI Qian, WU Fan, ZHAI Zhimin. Gene mutations and their relationship with clinical features in 100 patients with myelodysplastic syndrome [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(03): 305-312. |
| [5] | WANG Yanchun, LU Renquan. The application value of determination of hemostasis and thrombosis in tumor patients [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 341-347. |
| [6] | LI Lei, WU Xi, DAI Jing, WU Wenman, DING Qiulan, WANG Xuefeng. Clinical characteristics and risk factor analysis of 118 patients with cerebral venous sinus thrombosis [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(03): 261-269. |
| [7] | HAO Xu, WANG Weiming. Fabry disease presenting with renal disease as the main manifestation diagnosed by renal biopsy: a case report [J]. Journal of Diagnostics Concepts & Practice, 2022, 21(04): 527-529. |
| [8] | CAI Xiaoting, YI Huahua, LIN Jiayuan, CHEN Ling. Autosomal dominant polycystic kidney disease complicated with pulmonary embolism: a case report and literature review [J]. Journal of Diagnostics Concepts & Practice, 2022, 21(01): 80-85. |
| [9] | LEI Hang, FAN Liangfeng, CAI Xiaohong, WANG Yuqing, LIU Xi, JIN Sha, SHEN Wei, LU Qiong, XIANG Dong, WANG Xuefeng, ZOU Wei. The study on molecular basis of ABO blood subgroups in the Chinese population [J]. Journal of Diagnostics Concepts & Practice, 2020, 19(04): 364-369. |
| [10] | PENG Zhenping, XIANG Xixi, ZHANG Sujiang, LI Jiaming. Chronic neutrophilic leukemia with leukemia-like reaction as the first-onset manifestation: a report of 2 cases and literature review [J]. Journal of Diagnostics Concepts & Practice, 2020, 19(02): 122-128. |
| [11] | FENG Wei, ZHU Haohui. Value of color Doppler ultrasonography in evaluation of stenosis and thrombosis of autogenous arteriovenous fistula [J]. Journal of Diagnostics Concepts & Practice, 2019, 18(03): 360-364. |
| [12] | CAI Rong, MIN Xuewen, CHEN Meirong, SHEN Yating, SHI Qunli, ZHOU Xiaodie. Expression of BRAF V600E (VE1) in thyroid papillary carcinoma and its clinical significance [J]. Journal of Diagnostics Concepts & Practice, 2018, 17(05): 552-556. |
| [13] | WANG Dengfeng, CUI Wenyan, ZOU Wei, LI Fang, WANG Xuefeng, CAI Xiaohong. Molecular mechanism of Ax subtype caused by p.M142I mutation in alpha 1-3-N-acetylgalactosaminyltransferase [J]. Journal of Diagnostics Concepts & Practice, 2018, 17(03): 260-265. |
| [14] | LU Jing, XU Yufei, QING Yanrong, HAN Cong, LI Niu, YU Tingting, YAO Ruen, WANG Jian. Concurrent gene mutation analysis of a developmental delayed child with Rett syndrome and Noonan syndrome [J]. Journal of Diagnostics Concepts & Practice, 2018, 17(02): 147-150. |
| [15] | JIN Peipei, LIANG Qian, DAI Jing, DING Qiulan, SUN Shunchang, WANG Xuefeng. Phenotype and genotype analysis of a Chinese pedigree with 2N type von Willebrand disease [J]. Journal of Diagnostics Concepts & Practice, 2018, 17(02): 151-154. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||