

Journal of Diagnostics Concepts & Practice ›› 2024, Vol. 23 ›› Issue (05): 500-508.doi: 10.16150/j.1671-2870.2024.05.006
• Original articles • Previous Articles Next Articles
RUAN Miao, DA Qian, XU Haimin, DONG Lei, FEI Xiaochun( )
)
Received:2024-02-06
Accepted:2024-08-02
Online:2024-10-25
Published:2025-02-25
Contact:
FEI Xiaochun
E-mail:xcf0222@163.com
CLC Number:
RUAN Miao, DA Qian, XU Haimin, DONG Lei, FEI Xiaochun. Study on clinicopathological features and prognosis of HER2 low expression breast cancer[J]. Journal of Diagnostics Concepts & Practice, 2024, 23(05): 500-508.
Table 1
Summary of previous and reviewed results of HER2 IHC interpretation
| Previous result | Observer 1 | Observer 2 | Adjusted result | Complete agreement n(%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2 | n | HER2 | n | HER2 | n | HER2 | n | ||||
| 0 | 63 | 0 | 43 | 0 | 45 | 0 | 49 | 43(87.8) | |||
| 1+ | 20 | 1+ | 18 | ||||||||
| 1+ | 57 | 0 | 9 | 0 | 4 | 1+ | 69 | 36(52.2) | |||
| 1+ | 36 | 1+ | 49 | ||||||||
| 2+ | 12 | 2+ | 4 | ||||||||
| 2+ | 48 | 1+ | 3 | 1+ | 2 | 2+ | 48 | 40(83.3) | |||
| 2+ | 40 | 2+ | 44 | ||||||||
| 3+ | 5 | 3+ | 2 | ||||||||
| 3+ | 69 | 2+ | 1 | 2+ | 0 | 3+ | 71 | 68(95.8) | |||
| 3+ | 68 | 3+ | 69 | ||||||||
| Total | 187(78.9) | ||||||||||
Table 2
Factors contributing to interobserver inconsistency in HER2 interpretation
| Apparent causes | n(%) |
|---|---|
| Objective factors | 24(48.0) |
| Borderline expression | 21 |
| Intratumor heterogeneity of staining | 3 |
| Subjective factors | 14(28.0) |
| Previously no carefully evaluated in HPF | 12 |
| Observer error | 2 |
| Interference factors | 12(24.0) |
| Nonspecific staining of stroma | 7 |
| Cytoplasmic staining | 5 |
Figure 2
Three patterns of intratumor heterogeneity and HER2 score 0-2+ A: “Clustered”type with IHC 3+ on the left and 2+ on the right (×40); B: “Scattered”type with IHC 2+ tumor cells scattered among IHC 1+ (arrows, ×400); C: “Mosaic”type, HER2 IHC 3+ mixed with 2+ and 1+ (×200); D: HER2 score 0 (×400); E: HER2 score 1+ (×400); F: HER2 score 2+ (×200).
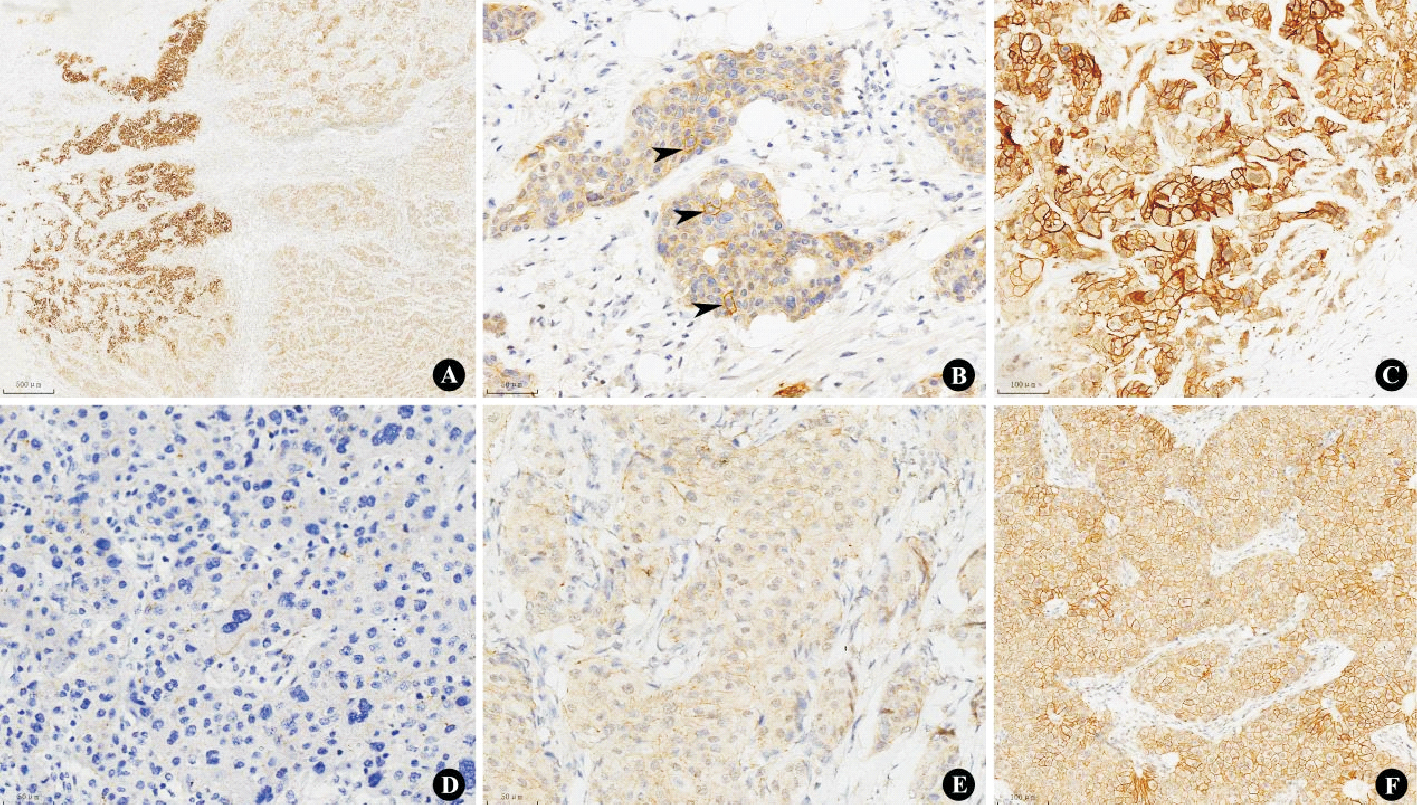
Table 3
Comparison of clinicopathologic features between HER2-low and HER2-negative/positive breast cancer
| HER2-low (n=113) | HER2-negative (n=49) | P value | HER2-positive (n=75) | P value | |
|---|---|---|---|---|---|
| Age (years) | 0.224 | 0.101 | |||
| ≤50 | 35(31.0) | 20(40.8) | 32(42.7) | ||
| >50 | 78(69.0) | 29(59.2) | 43(57.3) | ||
| Histological type | 0.231 | 0.774 | |||
| IBC, NST | 101(89.4) | 47(95.9) | 68(90.7) | ||
| Others | 12(10.6) | 2(4.1) | 7(9.3) | ||
| T stage | 0.464 | 0.003 | |||
| 1 | 59(52.2) | 31(63.3) | 23(30.7) | ||
| 2 | 53(46.9) | 18(36.7) | 48(64.0) | ||
| 3 | 1(0.9) | 0 | 4(5.3) | ||
| Histological grade | 0.045 | <0.001 | |||
| 1 | 13(11.5) | 4(8.2) | 0 | ||
| 2 | 57(50.4) | 16(32.6) | 17(22.7) | ||
| 3 | 43(38.1) | 29(59.2) | 58(77.3) | ||
| LVI | 0.400 | 0.764 | |||
| Positive | 25(22.1) | 8(16.3) | 18(24.0) | ||
| Negative | 88(77.9) | 41(83.7) | 57(76.0) | ||
| N stage | 0.285 | 0.516 | |||
| 0 | 85(75.2) | 39(79.6) | 52(69.3) | ||
| 1 | 16(14.2) | 8(16.4) | 15(20.0) | ||
| 2 | 11(9.7) | 1(2.0) | 6(8.0) | ||
| 3 | 1(0.9) | 1(2.0) | 2(2.7) | ||
| ER | 0.001 | <0.001 | |||
| Positive | 95(84.1) | 30(61.2) | 37(49.3) | ||
| Negative | 18(15.9) | 19(38.8) | 38(50.7) | ||
| PR | 0.001 | <0.001 | |||
| Positive | 88(77.9) | 26(52.9) | 27(36.0) | ||
| Negative | 25(22.1) | 23(47.1) | 48(64.0) | ||
| Ki-67 | 0.008 | <0.001 | |||
| ≤30% | 84(74.3) | 26(53.1) | 37(49.3) | ||
| >30% | 29(25.7) | 23(46.9) | 38(50.7) | ||
| Intrinsic subtype | 0.006 | <0.001 | |||
| Luminal A | 34(30.1) | 8(16.3) | 0 | ||
| Luminal B | 61(54.0) | 22(44.9) | 38(50.7) | ||
| HER2-enriched | 0 | 0 | 37(49.3) | ||
| TNBC | 18(15.9) | 19(38.8) | 0 |
Table 4
Comparison of clinicopathological characteristics between HER2-low and negative breast cancer after adjusting ER status
| Item | ER positive | ER negative | |||||
|---|---|---|---|---|---|---|---|
| HER2-low (n=95) | HER2-negative (n=30) | P value | HER2-low (n=18) | HER2-negative (n=19) | P value | ||
| Age (years) | 0.195 | 0.823 | |||||
| ≤50 | 29(30.5) | 13(43.3) | 6(33.3) | 7(36.8) | |||
| >50 | 66(69.5) | 17(56.7) | 12(66.7) | 12(63.2) | |||
| Histological type | 0.732 | 0.486 | |||||
| IBC, NST | 84(88,4) | 28(93.3) | 17(94.4) | 19(100) | |||
| Others | 11(11.6) | 2(6.7) | 1(5.6) | 0 | |||
| T stage | 0.125 | 0.362 | |||||
| 1 | 54(56.8) | 23(76.7) | 5(27.8) | 8(42.1) | |||
| 2 | 40(42.1) | 7(23.3) | 13(72.2) | 11(57.9) | |||
| 3 | 1(1.1) | 0 | 0 | 0 | |||
| Histological grade | 0.472 | 1.000 | |||||
| 1 | 13(13.7) | 4(13.3) | 0 | 0 | |||
| 2 | 55(57.9) | 14(46.7) | 2(11.1) | 2(10.5) | |||
| 3 | 27(28.4) | 12(40.0) | 16(88.9) | 17(89.5) | |||
| LVI | 0.614 | 0.693 | |||||
| Positive | 21(22.1) | 5(16.7) | 4(22.2) | 3(15.8) | |||
| Negative | 74(77.9) | 25(83.3) | 14(77.8) | 16(84.2) | |||
| N stage | 0.490 | 0.566 | |||||
| 0 | 72(75.8) | 24(80.0) | 13(72.2) | 15(78.9) | |||
| 1 | 11(11.6) | 5(16.7) | 5(27.8) | 3(15.8) | |||
| 2 | 11(11.6) | 1(3.3) | 0 | 1(5.3) | |||
| 3 | 1(1.0) | 0 | 0 | 0 | |||
| PR | 0.295 | / | |||||
| Positive | 88(92.6) | 26(86.7) | 0 | 0 | |||
| Negative | 7(7.4) | 4(13.3) | 18(100) | 19(100) | |||
| Ki67(%) | 0.591 | 0.405 | |||||
| ≤30 | 80(84.2) | 24(80.0) | 4(22.2) | 2(10.5) | |||
| >30 | 15(15.8) | 6(20.0) | 14(77.8) | 17(89.5) | |||
| Intrinsic subtype | 0.356 | / | |||||
| Luminal A | 34(35.8) | 8(26.7) | 0 | 0 | |||
| Luminal B | 61(64.2) | 22(73.3) | 0 | 0 | |||
| HER2-enriched | 0 | 0 | 0 | 0 | |||
| TNBC | 0 | 0 | 18(100) | 19(100) | |||
| [1] | SCHECHTER A L, STERN D F, VAIDYANATHAN L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen[J]. Nature, 1984, 312(5994):513-516. |
| [2] |
SLAMON D J, CLARK G M, WONG S G, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene[J]. Science, 1987, 235(4785):177-182.
doi: 10.1126/science.3798106 pmid: 3798106 |
| [3] | WOLFF A C, HAMMOND M E H, ALLISON K H, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update[J]. J Clin Oncol, 201, 36(20):2105-2122. |
| [4] | GIORDANO S H, FRANZOI M A B, TEMIN S, et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: asco guideline update[J]. J Clin Oncol, 2022, 40(23):2612-2635. |
| [5] | PONDÉ N, BRANDÃO M, EL-HACHEM G, et al. Treatment of advanced HER2-positive breast cancer: 2018 and beyond[J]. Cancer Treat Rev, 2018,67:10-20. |
| [6] |
SCHALPER K A, KUMAR S, HUI P, et al. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria[J]. Arch Pathol Lab Med, 2014, 138(2):213-219.
doi: 10.5858/arpa.2012-0617-OA pmid: 24164555 |
| [7] |
TARANTINO P, HAMILTON E, TOLANEY S M, et al. HER2-low breast cancer: pathological and clinical landscape[J]. J Clin Oncol, 2020, 38(17):1951-1962.
doi: 10.1200/JCO.19.02488 pmid: 32330069 |
| [8] |
BANERJI U, VAN HERPEN C M L, SAURA C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study[J]. Lancet Oncol, 2019, 20(8):1124-1135.
doi: S1470-2045(19)30328-6 pmid: 31257177 |
| [9] | MODI S, PARK H, MURTHY R K, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ⅰb study[J]. J Clin Oncol, 2020, 38(17):1887-1896. |
| [10] | MODI S, JACOT W, YAMASHITA T, et al. Trastuzumab deruxtecan in previously treated her2-low advanced breast cancer[J]. N Engl J Med, 2022, 387(1):9-20. |
| [11] |
HAMMOND M E, HAYES D F, WOLFF A C, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer[J]. J Oncol Pract, 2010, 6(4):195-197.
doi: 10.1200/JOP.777003 pmid: 21037871 |
| [12] | 《乳腺癌HER2检测指南》编写组. 乳腺癌HER2检测指南(2019版)[J]. 中华病理学杂志, 2019, 48(3):169-175. |
| Recommend by Breast Cancer Expert Panel. Guideline for HER2 detection in breast cancer, the 2019 version[J]. Chin J Pathol, 2019, 48(3):169-175. | |
| [13] |
DOWSETT M, NIELSEN T O, A'HERN R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group[J]. J Natl Cancer Inst, 2011, 103(22):1656-1664.
doi: 10.1093/jnci/djr393 pmid: 21960707 |
| [14] | MARCHIÒ C, ANNARATONE L, MARQUES A, et al. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond[J]. Semin Cancer Biol, 2021,72:123-135. |
| [15] | PEGRAM M D, LIPTON A, HAYES D F, et al. Phase Ⅱ study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment[J]. J Clin Oncol, 1998, 16(8):2659-2671. |
| [16] |
COBLEIGH M A, VOGEL C L, TRIPATHY D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease[J]. J Clin Oncol, 1999, 17(9):2639-2648.
doi: 10.1200/JCO.1999.17.9.2639 pmid: 10561337 |
| [17] | ROBERT N, LEYLAND-JONES B, ASMAR L, et al. Randomized phase Ⅲ study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer[J]. J Clin Oncol, 2006, 24(18):2786-2792. |
| [18] |
FERNANDEZ A I, LIU M, BELLIZZI A, et al. Examination of low ERBB2 protein expression in breast cancer tissue[J]. JAMA Oncol, 2022, 8(4):1-4.
doi: 10.1001/jamaoncol.2021.7239 pmid: 35113160 |
| [19] |
KARAKAS C, TYBURSKI H, TURNER B M, et al. Interobserver and Interantibody reproducibility of her2 immunohistochemical scoring in an enriched HER2-low-expressing breast cancer cohort[J]. Am J Clin Pathol, 2023, 159(5):484-491.
doi: 10.1093/ajcp/aqac184 pmid: 36856777 |
| [20] |
SCHETTINI F, CHIC N, BRASÓ-MARISTANY F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer[J]. NPJ Breast Cancer, 2021, 7(1):1.
doi: 10.1038/s41523-020-00208-2 pmid: 33397968 |
| [21] | THOMSON T A, HAYES M M, SPINELLI J J, et al. HER-2/neu in breast cancer: interobserver variability and performance of immunohistochemistry with 4 antibodies compared with fluorescent in situ hybridization[J]. Mod Pathol, 2001, 14(11):1079-1086. |
| [22] | AGOSTINETTO E, REDITI M, FIMERELI D, et al. HER2-low breast cancer: molecular characteristics and prognosis[J]. Cancers (Basel), 2021, 13(11):2824. |
| [23] | ZHANG H, KATERJI H, TURNER B M, et al. HER2-low breast cancers: incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles[J]. Mod Pathol, 2022, 35(8):1075-1082. |
| [24] | ZHANG H, PENG Y. Current biological, pathological and clinical landscape of HER2-low breast cancer[J]. Cancers (Basel), 2022, 15(1):126. |
| [25] |
GAMPENRIEDER S P, RINNERTHALER G, TINCHON C, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry[J]. Breast Cancer Res, 2021, 23(1):112.
doi: 10.1186/s13058-021-01492-x pmid: 34906198 |
| [26] |
ROSSO C, VOUTSADAKIS I A. Characteristics, clinical differences and outcomes of breast cancer patients with negative or low HER2 expression[J]. Clin Breast Cancer, 2022, 22(4):391-397.
doi: 10.1016/j.clbc.2022.02.008 pmid: 35337735 |
| [27] | TARANTINO P, JIN Q, TAYOB N, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cance[J]. JAMA Oncol, 2022, 8(8):1177-1183. |
| [28] |
DENKERT C, SEITHER F, SCHNEEWEISS A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials[J]. Lancet Oncol, 2021, 22(8):1151-1161.
doi: 10.1016/S1470-2045(21)00301-6 pmid: 34252375 |
| [29] |
ZHANG G, REN C, LI C, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status[J]. BMC Med, 2022, 20(1):142.
doi: 10.1186/s12916-022-02346-9 pmid: 35484593 |
| [30] | ALVES F R, GIL L, VASCONCELOS DE MATOS L, et al. Impact of human epidermal growth factor receptor 2 (HER2) low status in response to neoadjuvant chemotherapy in early breast cancer[J]. Cureus, 2022, 14(2):e22330. |
| [31] |
WON H S, AHN J, KIM Y, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society[J]. Breast Cancer Res, 2022, 24(1):22.
doi: 10.1186/s13058-022-01519-x pmid: 35307014 |
| [32] | DI COSIMO S, LA ROCCA E, LJEVAR S, et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world[J]. Front Mol Biosci, 2022,9:996434. |
| [33] | LI Y, ABUDUREHEIYIMU N, MO H, et al. In real life, low-level HER2 expression may be associated with better outcome in her2-negative breast cancer: a study of the National Cancer Center, China[J]. Front Oncol, 2022,11:774577. |
| [34] | KANG S, LEE S H, LEE H J, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy[J]. Eur J Cancer, 2022,176:30-40. |
| [35] | DE MOURA LEITE L, CESCA M G, TAVARES M C, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer[J]. Breast Cancer Res Treat, 2021, 190(1):155-163. |
| [36] | BARDIA A, HU X, DENT R, et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer[J]. Engl J Med. 2024,(391):2110-2122. |
| [1] | ZHANG Junhua, LI Yilin, XIE Jingyuan, ZHANG Chunli, XU Jing. Analysis of pathological features related to clinical prognosis in C3 glomerulopathy [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(06): 587-593. |
| [2] | WANG Yurong, WANG Yuanyuan, WENG Haiyan. Clinical and pathological analysis of gastrointestinal leiomyosarcoma:Report of three cases [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(05): 537-541. |
| [3] | LI Zhuohan, HUANG Xinyun, GUO Rui, LI Biao. 18F-FDG PET/CT in the diagnosis and prognosis evaluation of follicular lymphoma [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(04): 439-444. |
| [4] | ZHU Weiwei, LI Qian, WU Fan, ZHAI Zhimin. Gene mutations and their relationship with clinical features in 100 patients with myelodysplastic syndrome [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(03): 305-312. |
| [5] | NI Yaping, CHEN Yifeng, YANG Xiaoqun, CHEN Xiaoyan. Primary lung adenocarcinoma with enteroblastic differentiation: a clinicopathological and prognostic analysis of two cases [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(03): 324-329. |
| [6] | LIU Juan, YIN Lijuan, FAN Desheng. The clinicopathologic significance of AR, SKP2, SOX10, PD-L1 and TILs expression in triple-negative breast cancer [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(02): 162-172. |
| [7] | OU Dan, CAI Gang, CHEN Jiayi. Bioinformatics analysis for expression of RAD51AP1 in triple negative breast cancer with brain metastasis [J]. Journal of Diagnostics Concepts & Practice, 2024, 23(02): 146-154. |
| [8] | WANG Shukui, GU Xinliang. Advances in the study of tsRNA as diagnostic and prognostic biomarkers in cancer [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(05): 413-420. |
| [9] | LIU Yingting, YI Hongmei, WANG Xue, YANG Chunxue, OUYANG Binshen XU Haimin, WANG Chaofu. Clinicopathological features and prognosis of 17 cases of duodenal-type follicular lymphoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 362-368. |
| [10] | ZHANG Lanlan, YANG Qiao, NIE Zunzhen, GUO Ying. Thoracic SMARCA4-deficient undifferentiated tumour: a case report [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 389-392. |
| [11] | HU Jingjing, SHEN Yinzhong, LIU Li, LU Hongzhou. Current status and research progress of diagnosis and treatment of AIDS with disseminated non-tuberculous mycobacterial disease [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(04): 402-406. |
| [12] | XU Li, GAO Huajie, YANG Mengge, LI Yue, JI Suqiong. Clinical characteristics of anti-SRP antibody positive immune-mediated necrotizing myopathy with anti-TRIM21/Ro52 antibody positive [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(03): 247-254. |
| [13] | ZHOU Xiaodie, CHEN Weiwei, YU Bo, WANG Xuan, WANG Jianjun, SHI Qunli, RAO Qiu, BAO Wei. Clinicopathological features of urothelial carcinoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(03): 292-299. |
| [14] | SONG Luqian, CHANG Chunkang. Interpretation of clinical practice guidelines for myelodysplastic syndrome (version 1, 2023) of National Comprehensive Cancer Nerwork(NCCN) [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(02): 116-120. |
| [15] | XU Jiankun, ZHOU Luting, ZHANG Wenjing, XU Haimin, WANG Chaofu. The prognostic value of CA9 expression in clear cell renal cell carcinoma [J]. Journal of Diagnostics Concepts & Practice, 2023, 22(01): 37-43. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||